Hepatic stellate cells account for 33% of the total number of liver nonparenchymal cells and 1.4% of the total volume of the liver, and are adherent growth cells of a single layer, with star-shaped, spindle-shaped, polygonal, fusiformal, pseudopodia, and more lipid droplets in the cytoplasm, also known as hepatic fat storage cells.
Hepatic stellate cells have a variety of important functions in the liver and are involved in most pathophysiological processes such as fibrosis, inflammation and immune disorders in the liver, and studying hepatic stellate cells can help us better understand the function of the liver in healthy and disease states, especially in pathological processes such as fibrosis, inflammation, immune regulation, and liver cancer. Therefore, in-depth study of the mechanism of action of hepatic stellate cells is essential for the treatment of liver diseases.
Preparation before the experiment
●Pre-warm perfusion buffer and digestion buffer:
Pre-warming the Perfusion buffer and Digestion buffer in a thermostatic water bath at 42°C ensures that the solution remains at a constant temperature during perfusion, helping to improve perfusion and digestion efficiency.
Preparation Before Experiment
●Pre-warm perfusion buffer and digestion buffer:
Pre-warming the Perfusion buffer and Digestion buffer in a thermostatic water bath at 42°C ensures that the solution remains at a constant temperature during perfusion, helping to improve perfusion and digestion efficiency.
●Peristaltic pump disinfection:
Wash the peristaltic pump tubing with 70% ethanol to maintain the sterility of the peristaltic pump. The disinfection time is at least 5 minutes to ensure thorough disinfection.
After disinfection, rinse the peristaltic pump tubing with sterile saline to remove residual ethanol and avoid interference with subsequent experiments.
●Ligation perfusion buffer:
The preheated perfusion buffer is connected to the peristaltic pump and the pump is activated to drain air from tube, ensuring a smooth flow of liquid and preventing air embolus from affecting the perfusion.
Mouse anesthesia with intraperitoneal exposure:
●Mouse anesthesia:
Fully anesthetize the mouse with an appropriate anesthetic (such as isoflurane or sodium pentobarbital) to ensure pain-free throughout the procedure.
After confirming the anesthetic effect, fix the mouse on the operating table with its back facing down.
Hepatic perfusion:
●Needle perfusion:
Carefully insert the hepatic portal vein with a perfusion needle and secure the needle with a vascular clip to ensure that the needle is secure and does not slip. At this time, the hepatic portal vein is the main passage into the liver, and the needle should be punctured accurately to avoid puncturing the blood vessel too deeply.
●Start the peristaltic pump:
Turn on the peristaltic pump to initiate the flow of perfusion buffer. Slowly increase the flow rate to ensure smooth fluid flow into the liver without causing tissue damage.
Watch for changes in the liver and inferior vena cava: If there is slight swelling in the liver and inferior vena cava, fluid has begun to perfuse into the liver.
●Shear the inferior vena cava:
When swelling of the inferior vena cava and liver is observed, cut the inferior vena cava with sterile scissors to drain excess blood and perfusion. The shear site should be as far away from the liver end as possible to prevent damage to the liver tissue.
●Assisted perfusion:
During perfusion, the inferior vena cava can be clamped appropriately to increase the perfusion pressure and help the perfusion fluid pass more evenly through all areas of the liver to ensure adequate perfusion.
●End of perfusion:
Perfusion is continued until the liver is visibly white, indicating that the blood has been largely emptied and the perfusate has completely entered the liver. This process usually takes 2-3 minutes.
Liver digestion steps
●Replacement of the digestive solution:
At the end of the hepatic perfusion process, perfusion is briefly stopped and the perfusion tubing is immediately removed from the perfusion buffer and quickly connected to the pre-warmed digestion buffer. Make sure that the digest solution is pre-warmed to the appropriate temperature (usually 37 °C) to keep enzyme activity.
●Digestive fluid perfusion:
The peristaltic pump is activated to begin perfusing the liver with the digestive juices. The inferior vena cava can be clamped appropriately to help the digestive juices enter the liver tissue more fully and ensure that the enzymes are evenly distributed throughout the liver.
●Digestion process:
Observe changes in liver tissue. During digestion, the liver tissue should gradually become soft and cracked. This is because digestive enzymes are breaking down the extracellular matrix in the tissues, making the liver tissue more potent.
Usually, the digestion process takes 15-20 minutes. During this time, the state of the liver tissue is checked regularly to ensure that digestion is even and effective. Over-digestion may lead to cell damage, so care needs to be taken to control the time.
●Liver tissue extraction:
Digestion ends when there is a visible crack in the liver tissue, indicating that digestion is almost complete. Carefully remove the liver tissue to avoid damaging the tissue.
Excise accessory structures such as the gallbladder with sterile scissors and forceps. Ensure that all gallbladder tissue is completely removed so as not to affect the subsequent isolation process.
●Transfer to a centrifuge tube:
Place the treated liver tissue into a 50mL centrifuge tube containing digestion solution. Ensure that the digestion fluid in the centrifuge tube is sufficient to cover the liver tissue to maintain the stability of the tissue during isolation and centrifugation.
Gently shake the tube to ensure that the liver tissue and digestive juices are well mixed in preparation for the next step of cell isolation.
Hepatic stellate cell isolation steps
●Filter cell suspension:
User a 70 μm strainer to filter the cell suspension obtained from the liver tissue digestion solution. This step is to remove larger tissue debris and undigested particles and collect the filtered cell filtrate for later use.
●Low-speed centrifugation:
Transfer the cell filtrate to a centrifuge tube and centrifuge at 580 x g for 10 min at 4 °C using a low-speed refrigerated centrifuge at a relative centrifugal force of 580 x g. This step helps to pellet the cells while removing lytic material from the supernatant.

●Wash cells:
Carefully remove the supernatant, preserve 10 mL supernatant, add GBSS buffer into it to 50 mL, remix, and centrifuge again at 580 x g for 10 min at 4 °C. This step is to remove residual extracellular matrix and impurities and wash the cells.
●Resuspend cells:
Remove the centrifuged supernatant, preserve 10 mL supernatant, and resuspend the cells in 10 mL GBSS buffer and add to a total volume of 20 mL and mix the cell suspension gently.
●Density gradient centrifugation:
Add 10 mL of Nycodenz medium (a density gradient medium) to the cell suspension, mix well, and aliquot the mixture into three 15 mL centrifuge tubes, making sure that the walls of the tubes are adequately rinsed to help cell sedimentation.
Use a 3 mL syringe to gently add 1.5 mL of GBSS buffer as blocking solution to establish a density gradient to ensure efficient separation.
●Centrifugal stratification:
Place the centrifuge tubes in a centrifuge and centrifuge at 4 °C using 1,380 x g for 17 min without brakes. This process stratifies the cells, with a white layer in the middle that contains hepatic stellate cells.
●Collection of stellate cell layers:
At the end of centrifugation, carefully collect the middle white cell layer with a 5 mL pipette tip and transfer to a new 50 mL centrifuge tube. Avoid perturbing the other layers and ensure that the fraction that is collected is rich in stellate cells.
●Final washing:
The collected cell suspension was replenished to 50 mL and centrifuged at 4 °C for 10 min using 580 x g for further cell purification.
●Resuspend cells and culture:
Remove the supernatant after centrifugation, resuspend the cells using a suitable medium (e.g., DMEM/F12 medium) and transfer the cell suspension to a flask, typically at 37 °C, 5% CO₂.
Once the cells are adherent, microscopic examination is performed to observe cell morphology and confirm cell purity.
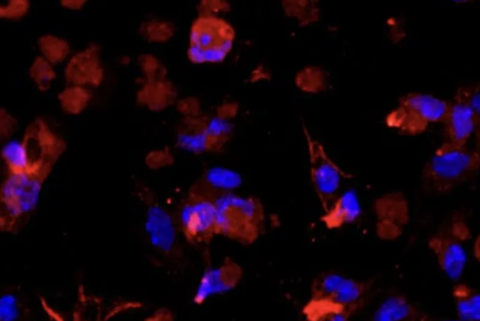
The isolated mouse hepatic stellate cells were identified by α-SMA or Desmin immunofluorescence, and the purity can reach more than 90% and can be used for downstream experiments (can be passed for about 3 generations).

Precautions
●Maintain aseptic procedures:
It is important to maintain a sterile environment and use sterile tools and reagents throughout the procedure. Benches, centrifuge tubes, and other utensils should be treated with 70% ethanol or other appropriate disinfectant in advance to prevent cell contamination.
Use aseptic techniques, such as wearing sterile gloves and using a sterile workbench to reduce contamination risks.
●Gentle operation:
Each step should be as gentle as possible to reduce mechanical damage to the cells. For example, when transferring cell suspensions and resuspending cells, avoid vigorous shaking or agitation.
When using a pipette or syringe, avoid direct contact with the cell pellet to reduce stress and shear forces on the cells.
●Centrifuge settings:
Make sure the temperature of the centrifuge is set correctly and keep it at 4 °C during centrifugation. Centrifuge temperature fluctuations can affect the physiological state of cells and even lead to cell damage or death.
Calibrate the centrifuge regularly and check the temperature control system to make sure it is working properly.
●Cell resuspension and culture:
During cell resuspension and culture, excessive agitation or shaking should be avoided. Cell suspensions should be mixed in a gentle upside-down or slow rotation to reduce physical damage to the cells.
During the culture process, avoid frequent stirring or shaking of the culture flask, and keep the culture environment stable to ensure the viability and function of the cells.
●Operation Details:
During isolation and washing, carefully observe the cell status at each step to ensure that the procedure is accurate. Any abnormalities (e.g., uneven cell sedimentation or bubble formation) should be adjusted promptly.
Use clean tools and disinfectants during operation to keep the experimental environment clean.
●Sample Recording and Management:
Record detailed information of each step, such as centrifugation time, speed, temperature, and other parameters, for subsequent analysis and result tracking.
Properly manage cell samples including labeling and storage conditions, to prevent sample loss or mix-up.
By strictly adhering to these precautions, it can ensure success rate of cell isolation and the quality of cell samples so as to provide reliable data support for subsequent experiments and research.
When isolating hepatic stellate cells, low-speed refrigerated centrifuge is the most suitable choice
●Temperature control function:
Keeping temperatures: Low-speed refrigerated centrifuges are able to operate at temperatures as low as 4 °C which is important for maintaining cell viability and reducing temperature fluctuations. For cells, especially hepatic stellate cells, temperature stability helps to avoid cell damage caused by heat accumulation.
●Low-speed centrifugation:
Reduced mechanical stress: Low-speed refrigerated centrifuges allow operation at lower centrifugal forces such as 580 x g or 1380 x g, which reduces shear and mechanical stress on the cells, protecting the integrity and function of the cells.
●Precise separation:
Density gradient centrifugation: Low-speed refrigerated centrifuges are suitable for density gradient centrifugation which is commonly used cell separation techniques. It enables stratification by different centrifugation speeds helping to isolate cells of interest (e.g., hepatic stellate cells) and remove contamination from other cell types.
In summary, although a low-speed refrigerated centrifuge is not the only isolation option, it can effectively improve the quality and purity of cell isolation by providing temperature control and gentle handling of cells when isolating hepatic stellate cells. If experimental conditions allow, the use of a low-speed refrigerated centrifuge is an ideal option to ensure the viability and accuracy of the cell sample.
